
- Home
-
Services
 Leading molecular discovery services
Leading molecular discovery services
 Antibody Discover Service
Antibody Discover Service
 Technology platform
Technology platform
 Technology optimization service
Technology optimization service
- Products
- Resources
- Publication
- Contact Us

 Leading molecular discovery services
Leading molecular discovery services Antibody Discover Service
Antibody Discover Service Technology platform
Technology platform Technology optimization service
Technology optimization service
Membrane proteins are extremely attractive as targets for research, diagnostic and therapeutic
applications.A critical factor in the successful isolation of new antibodies is the presentation
of a correctly folded antigen.
Partner with us to harness the untapped potential of membrane proteins for breakthrough innovations in
drug discovery and therapeutic development.
Membrane Protein Single-domain Antibody Development
Membrane proteins, including ion channels, transporters, G-protein-coupled receptors (GPCRs), and kinases, are the largest class of drug targets, with nearly 50% of approved drugs acting on them. Despite their critical role in biological pathways and diseases, their use in drug discovery is often hindered by:
· Low Expression Levels – Limited protein yield.
· Insolubility & Stability Issues – Difficulty maintaining solubility and native conformation.
· Purification Challenges – Complex isolation of functional proteins.
To overcome these challenges, we have developed the Membrane Protein Single-Domain Antibody Development Platform, designed to facilitate high-quality antibody drug discovery targeting membrane proteins.
Service Features
1. Diversified Immunization Platform
Our platform is optimized to tackle membrane protein challenges, supporting the discovery of high-affinity antibodies against these complex targets.
Immunogen Formats:
· Overexpression cell lines
· Plasmids & Virus-like Particles (VLPs)
· Micelles, Nanodiscs, SMALPs, & Salipro systems
· Adenoviruses & mRNA-based techniques
2. Proprietary Camelid Immune Adjuvant
Enhances immune response, increases HcAb titers, preserves antigen conformation, and maintains low toxicity.
Service Process
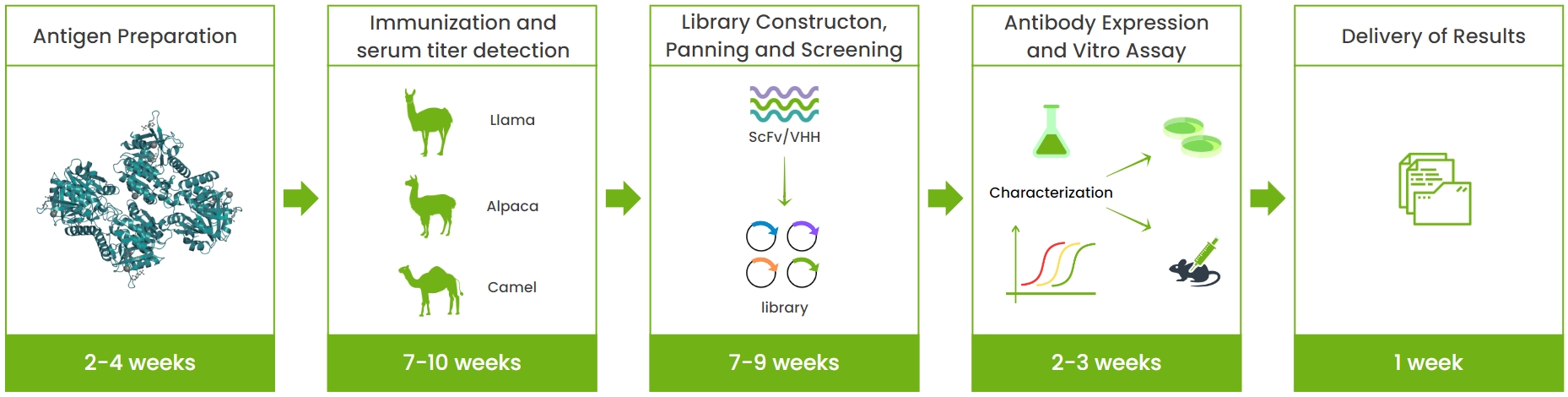
Service Advantages

Case Studies
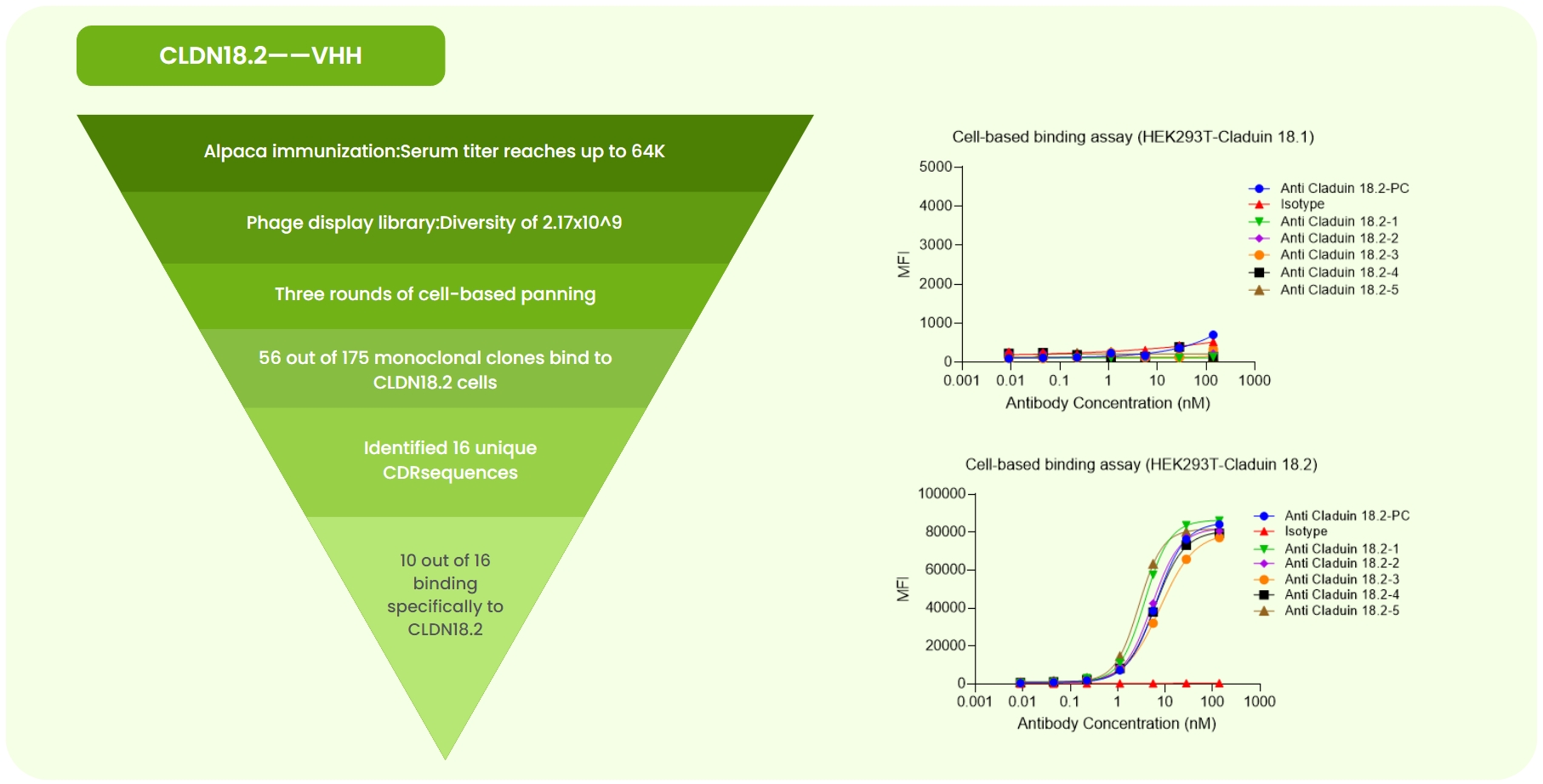
Fig 1. CLDN18.2 Single-Domain Antibody Development. (a) Immunized alpaca ELISA at 1:64K dilution showed OD >0.2. A 2.17×10⁹ phage library was constructed, followed by three rounds of cell panning. Sanger sequencing of 175 monoclones identified 16 unique CDR sequences. Expression and validation yielded 10 antibodies with high CLDN18.2 specificity. (b) Flow cytometry showed no or weak binding of anti-CLDN18.2-PC (benchmark), isotype, and five candidates to CLDN18.1-overexpressing cells. (c) Strong binding was observed for the same antibodies to CLDN18.2-overexpressing cells.
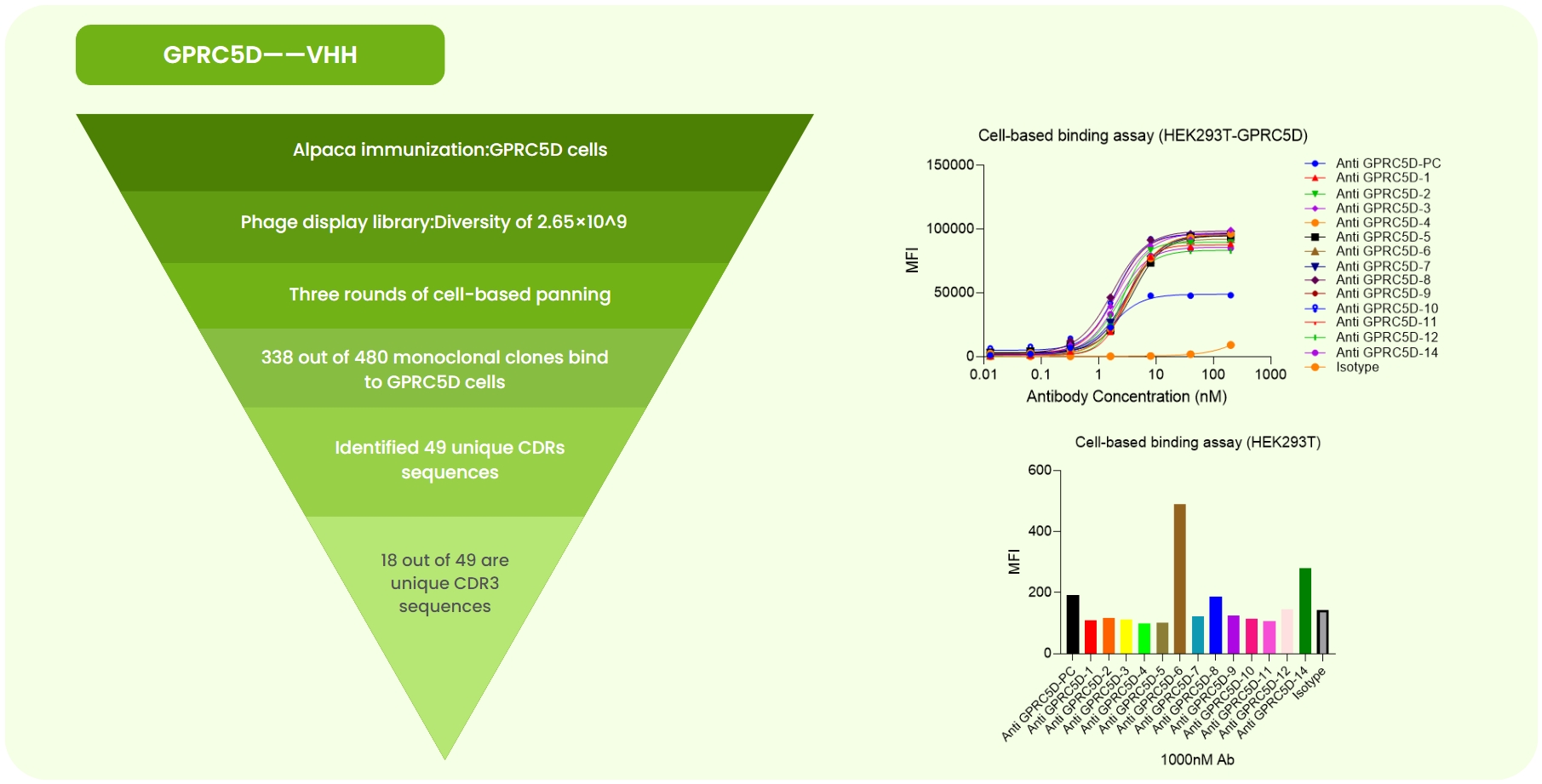
Fig 2. GPCR5D Single-Domain Antibody Development. (a) Alpaca immunized with GPCR5D-expressing cells. A 2.65×10⁹ phage library was constructed, followed by three rounds of cell panning. Sanger sequencing of 480 monoclones identified 49 unique CDR sequences. Expression and validation yielded 18 high-specificity antibodies. (b) Flow cytometry showed superior affinity of 13 candidates compared to the anti-GPCR5D-PC (benchmark) on GPCR5D-overexpressing cells. (c) At 100 nM, flow cytometry revealed varying binding strengths among candidates.

Tel:+86 4008677715
E-mail:service@nb-biolab.com
Address:SME Incubation Park, 319 Qingpi Avenue, Chengdu, China.
Working time:9:00-18:00