
- Home
-
Services
 Leading molecular discovery services
Leading molecular discovery services
 Antibody Discover Service
Antibody Discover Service
 Technology platform
Technology platform
 Technology optimization service
Technology optimization service
- Products
- Resources
- Publication
- Contact Us

 Leading molecular discovery services
Leading molecular discovery services Antibody Discover Service
Antibody Discover Service Technology platform
Technology platform Technology optimization service
Technology optimization service
Antibody Humanization Service
Immunogenicity is the ability of an antigen to trigger an immune response, influencing the safety and efficacy of antibody-based therapies. In drug development, many promising candidates fail due to reduced efficacy or adverse reactions caused by anti-drug antibodies. Humanizing candidate antibodies is crucial to overcoming these challenges.
Antibody humanization converts non-human antibodies into therapeutic drugs. While complementary-determining region (CDR) grafting is a common method, it alone does not fully eliminate immunogenicity risks. Factors such as activity, stability, and immunogenicity must be carefully balanced to ensure drug viability. We offer high-quality antibody humanization services using homology modeling, CDR grafting, back-mutation design, immunogenicity prediction, and PTM analysis.
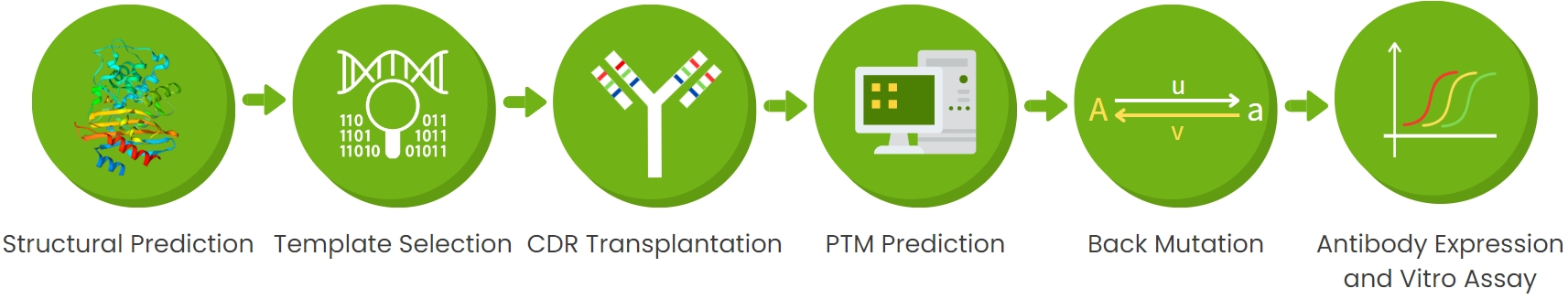
Fig 1. Antibody humanization service process

Case Study
Table 1. Humanization of a Single-Domain Antibody
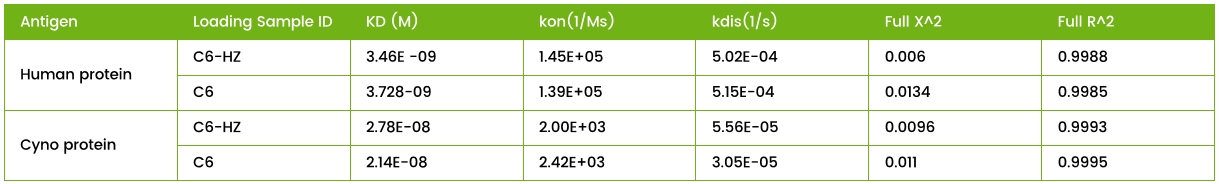
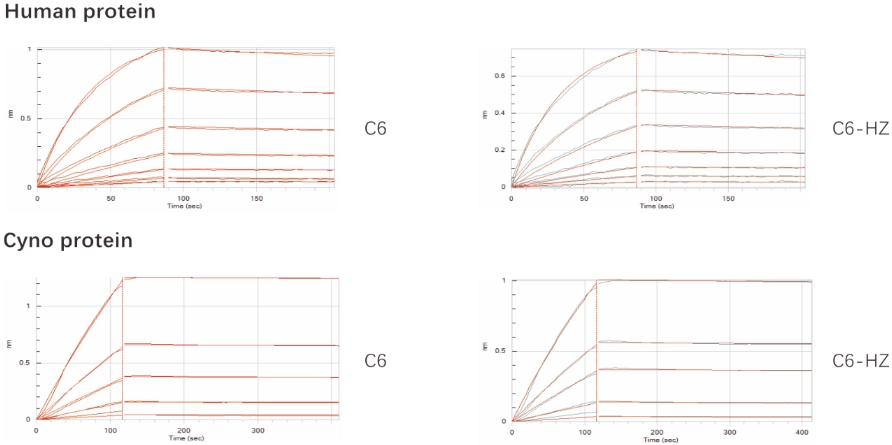
Fig 2. Cross-Species Binding of C6 Antibody Before and After Humanization. C6 antibody binding to human and cyno monkey proteins was evaluated. Affinity reduction after humanization did not exceed threefold.

Tel:+86 4008677715
E-mail:service@nb-biolab.com
Address:SME Incubation Park, 319 Qingpi Avenue, Chengdu, China.
Working time:9:00-18:00