
- Home
-
Services
 Leading molecular discovery services
Leading molecular discovery services
 Antibody Discover Service
Antibody Discover Service
 Technology platform
Technology platform
 Technology optimization service
Technology optimization service
- Products
- Resources
- Publication
- Contact Us

 Leading molecular discovery services
Leading molecular discovery services Antibody Discover Service
Antibody Discover Service Technology platform
Technology platform Technology optimization service
Technology optimization serviceAntibody Drugability Assessment
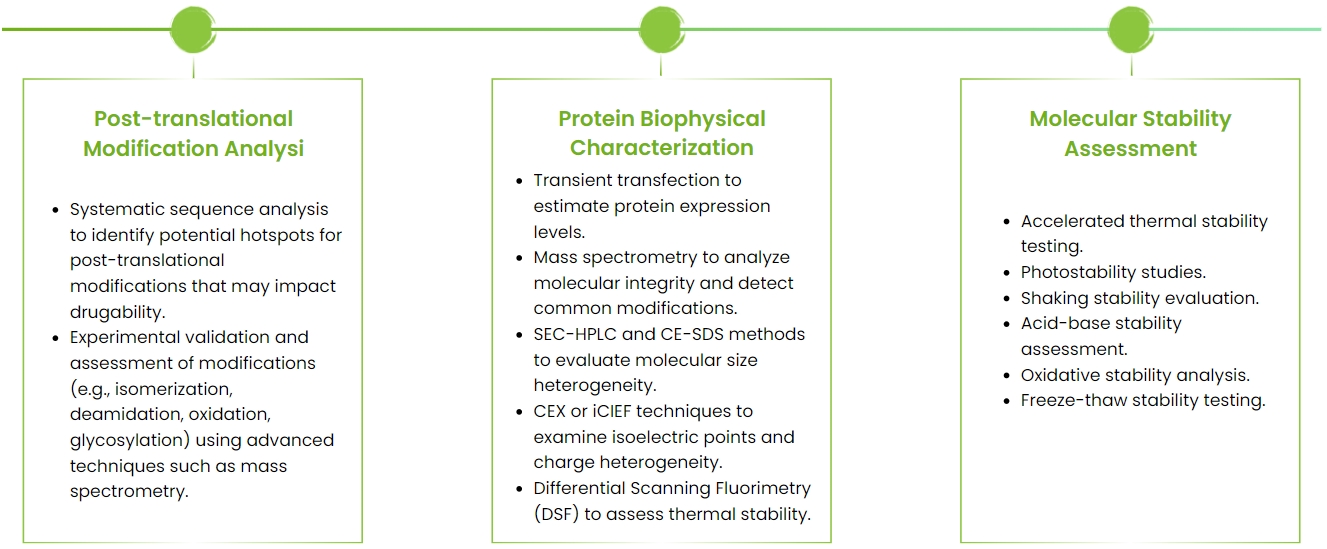
Antibody drugability is determined by its sequence, structure, and target-specific factors. The assessment includes both physicochemical and biochemical evaluations.
Physicochemical assessment focuses on colloidal stability, conformational stability, and environmental effects, using methods like AC-SINS, CSI-BLI, CIC, HIC, SGAC-SINS, and SEC-HIC to evaluate molecular interactions, aggregation, and viscosity.
Biochemical evaluation targets chemical reactions that may alter the structure, such as deamidation, isomerization, and oxidation of methionine and tryptophan, which could affect efficacy and immunogenicity.
NBbiolab provides comprehensive antibody drugability assessments, addressing immunogenicity, post-translational modifications, stability, and aggregation. We help optimize molecular performance, reduce development risks, and deliver high-quality preclinical antibody candidates.

Tel:+86 4008677715
E-mail:service@nb-biolab.com
Address:SME Incubation Park, 319 Qingpi Avenue, Chengdu, China.
Working time:9:00-18:00