
- Home
-
Services
 Leading molecular discovery services
Leading molecular discovery services
 Antibody Discover Service
Antibody Discover Service
 Technology platform
Technology platform
 Technology optimization service
Technology optimization service
- Products
- Resources
- Publication
- Contact Us
TCRm Single-domain Antibody Development Service
Antibody-based immunotherapies have revolutionized cancer treatment, demonstrating remarkable clinical efficacy. However, their potential is largely limited to targeting surface or soluble antigens, which constitute only a small fraction of the cancer proteome. A significant challenge in oncology is that the majority of proteins are intracellular, inaccessible to conventional antibodies.
TCR-mimicking (TCRm) single-domain antibodies overcome this limitation by targeting intracellular antigens presented on the surface of tumor cells as peptide-MHC class I (pMHC) complexes. By mimicking the natural recognition mechanisms of T-cell receptors (TCRs), these antibodies expand the therapeutic horizon of cancer immunotherapy.
· Broad Immune Activation: Triggering mechanisms such as antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP).
· Versatility: Transforming into chimeric antigen receptor (CAR) structures for targeted T-cell therapy against tumor cells.
Key Innovation in pMHC Complex Engineering
The stability of the peptide-MHC complex is crucial for successful antibody development. Our advanced approach links the MHCI heavy chain, β2-microglobulin (B2M), and target peptide through a linker, forming recombinant fusion proteins that mimic native pMHC complexes. These fusion proteins ensure accurate antigen presentation and robust antibody selection.
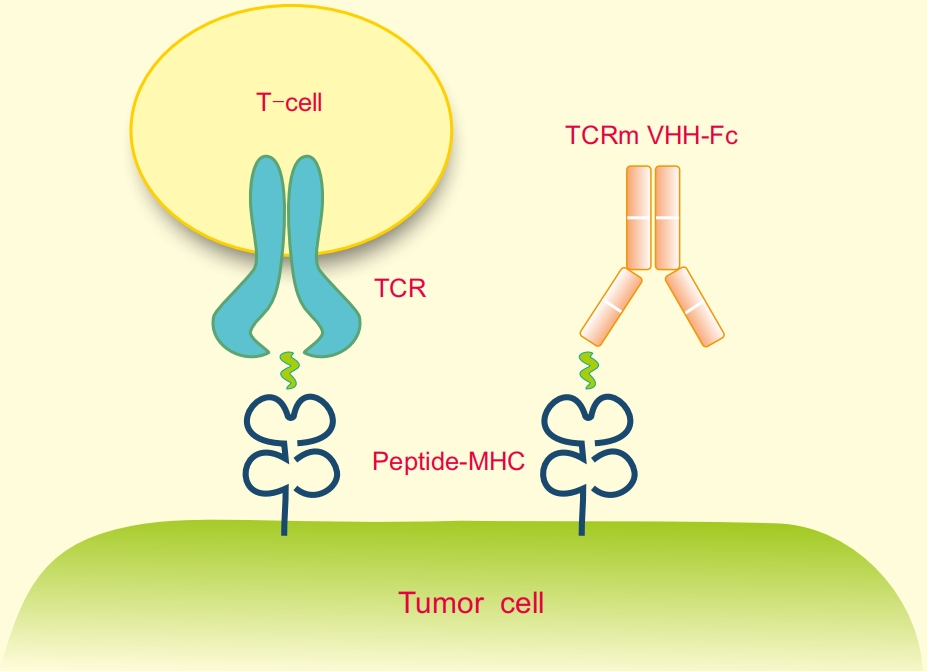
Fig 1. Mechanism of action of TCRm single-domain antibody development
Service Process
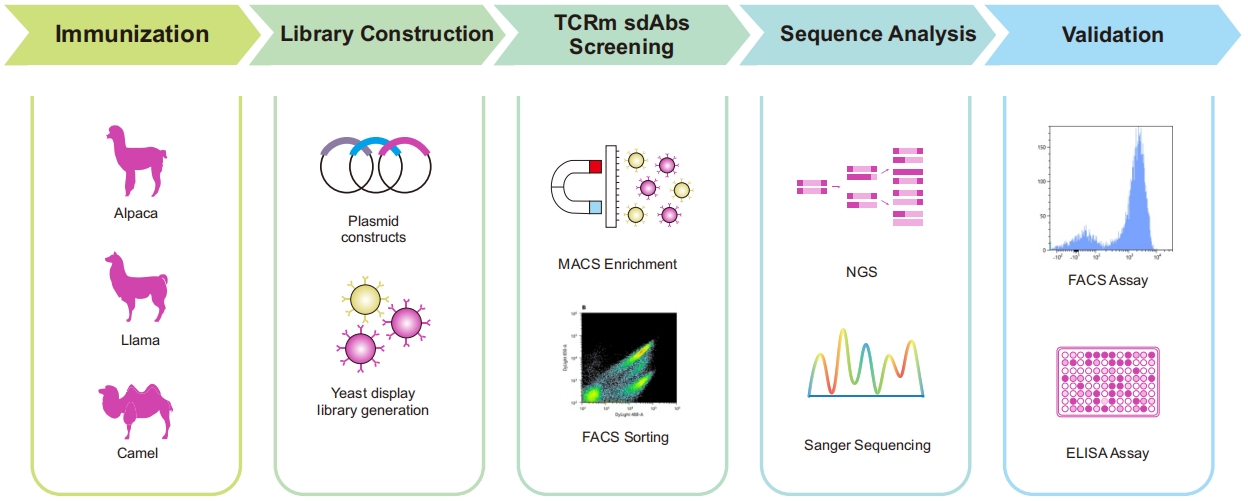
Fig 2. TCRm single-domain antibody development Service process based on yeast surface display platform
Our Expertise in TCRm Antibody Development
Traditional techniques like phage display and hybridoma technology are often inefficient for TCRm antibody discovery. Our platform leverages high-throughput yeast display technology to streamline the process, offering:
· High-Quality Library Construction: Optimized with yeast surface display for superior antibody generation.
· Precise Screening Control: Enabled by flow cytometry, allowing fine-tuned parameter adjustments.
· Advanced NGS Analysis: Enhancing antibody identification with cutting-edge sequencing insights.
· Proven Track Record: Extensive project experience ensures high success rates.
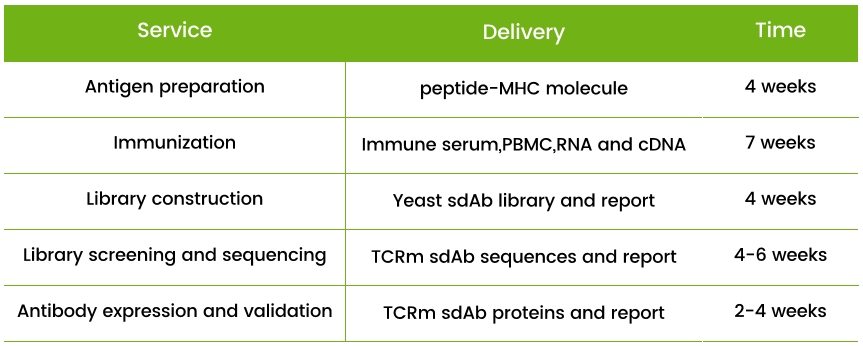
Service provided
Case Study
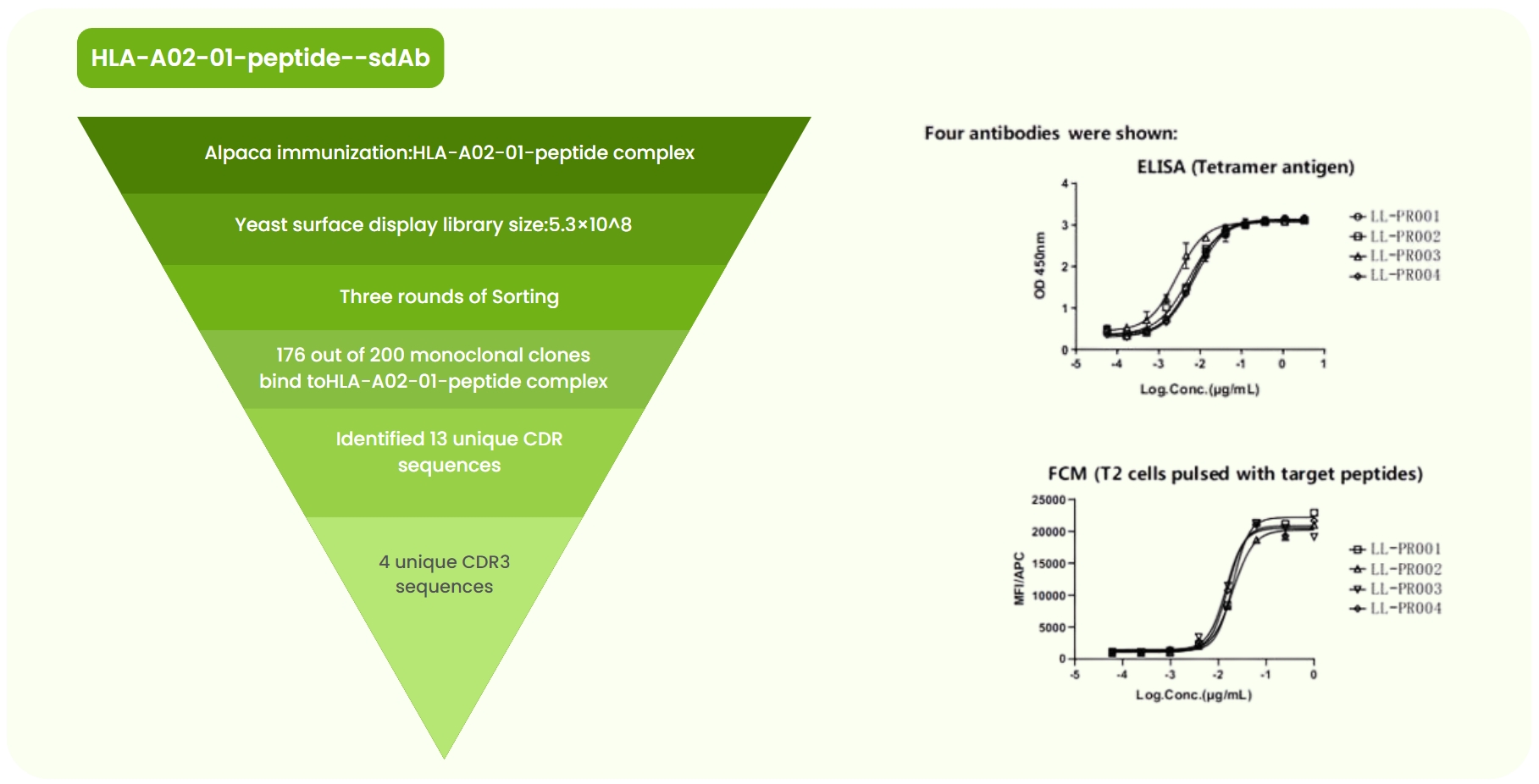
Fig 3. HLA-A02-01-peptide Single-Domain Antibody Development. (a) Aalpaca immunized with the HLA-A02-01-peptide complex. A yeast surface display library (5.3 × 10⁸) was constructed, followed by three rounds of sorting. Sanger sequencing of 200 monoclones identified 13 unique CDR sequences. Four antibodies with high specificity for HLA-A02-01-peptide were obtained. (b) ELISA confirmed strong binding signals of the four candidate antibodies to HLA-A02-01-peptide. (c) Flow cytometry showed strong binding of the four antibodies to T2 cells pulsed with the target peptide.