VHH, the variable region of a heavy-chain antibody, is the smallest antigen-binding domain with a molecule weight of only 1/10 of conventional antibodies(150-160 kDa), hence the name “nanobody”. VHHs are even smaller than Fab fragments (about 50 kDa, one light chain and half a heavy chain) and scFv fragments (about 25 kDa, two variable domains, one from the light chain and one from the heavy chain).
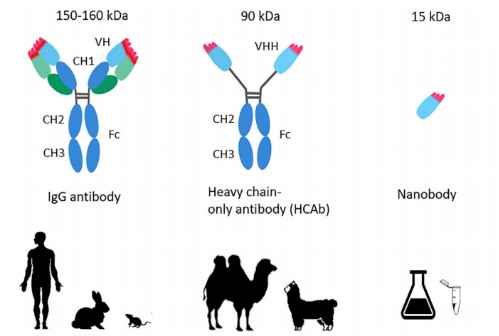
Figure 1. Conventional antibodies, heavy chain antibodies and nanobody(VHH)
VHHs have many advantages over traditional antibodies or antibody fragments such as scFvs or Fabs.
Better targeting
Compared to monoclonal antibodies, VHHs are made of the antigen-binding fragment of HcAbs and also retain full antigen-binding activity. Due to their small sizes (~15kDa), VHHs can bind hidden epitopes not accessible to whole antibodies. , so the targeting precision is higher compared to normal antibodies.
Easier production
Because VHHs are composed of one polypeptide chain, VHHs are relatively easy to produce in lower organisms (E. coli, yeast) up to very high amounts and purities. In addition, the hydrophilic side of nanobodies, which is not present in conventional antibodies, means they do not have issues with solubility and aggregation otherwise associated with conventional antibodies.
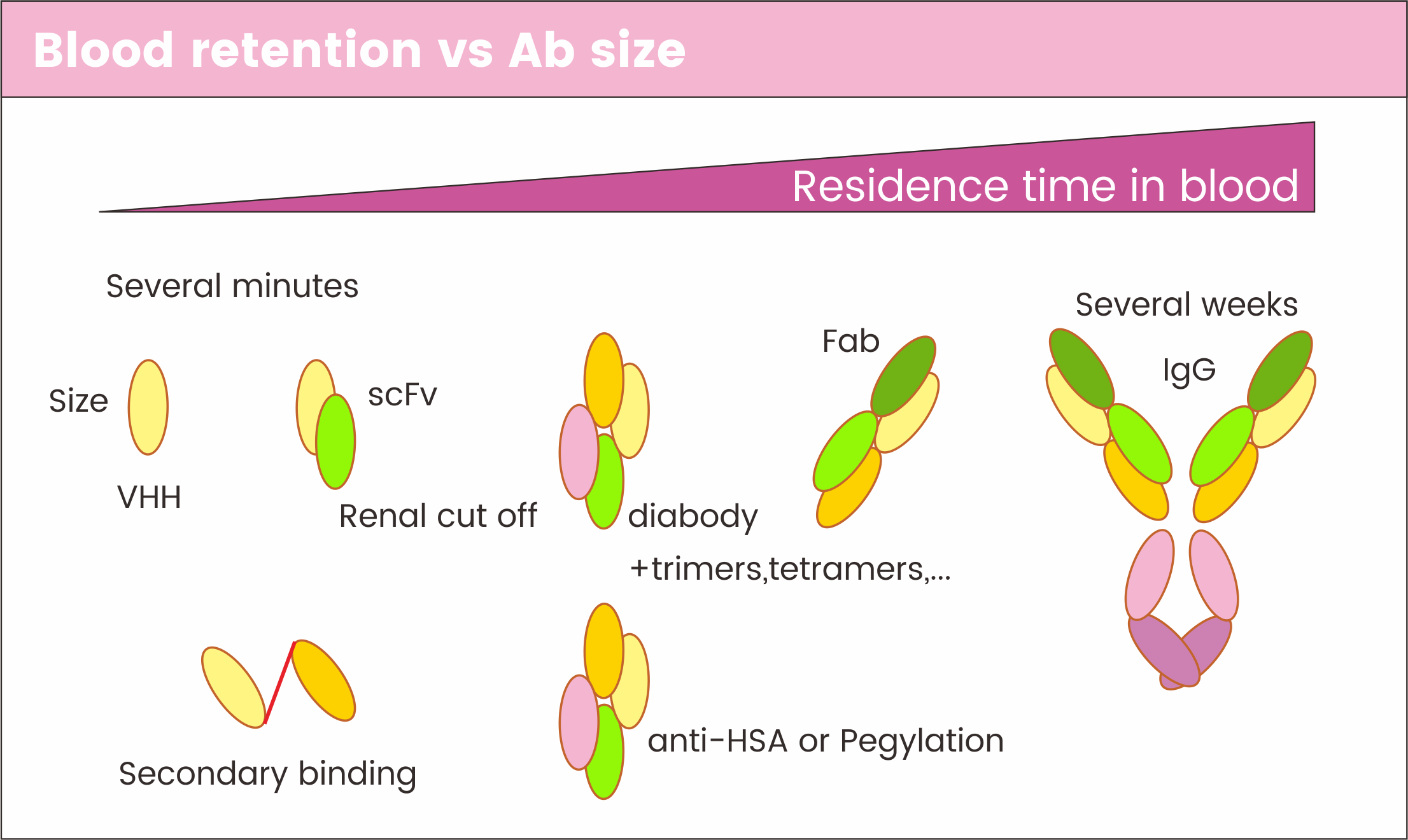
Better diffusion
VHHs have a high tissue penetration and are cleared from circulation rapidly. Some VHHs can even cross the blood-brain barrier.
Higher stability
VHHs are stable under a wide range of temperatures, extreme pH levels, and against proteases; hence, they keep their native folding and epitope binding capacity under very diverse experimental conditions.



Tel:+86 4008677715
E-mail:service@nb-biolab.com
Address:SME Incubation Park, 319 Qingpi Avenue, Chengdu, China.
Working time:9:00-18:00