Naturally occurring heavy-chain only antibodies (HcAbs) were accidentally discovered in camels by immunologist Raymond Harmers in 1989. After a few years of research, Harms confirmed this unexpected discovery and described them in Nature in 1993 (Hamers-Casterman et al., 1993).
HcAbs are a feature of all camelids. In addition to the conventional IgG1 composed of two identical heavy chains and two identical light chains, camelids' functional IgG2 and IgG3 lack the light chain and are composed of heavy-chain (lack CH1) dimers. The antigen-binding fragments of HcAbs in camelids are called VHHs, sdAbs or nanobodies. Cartilaginous fishes also have heavy-chain antibodies (IgNAR, 'immunoglobulin new antigen receptor'), from which antigen-binding fragments are called VNARs(Andrew S. Greenberg et al., 1995).
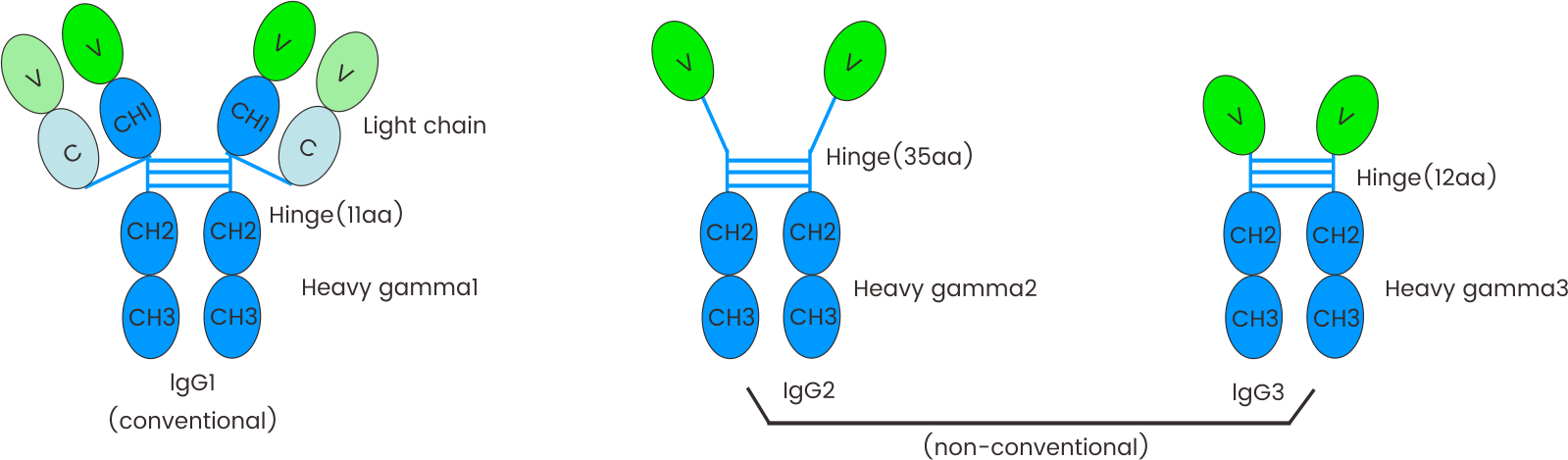
FIGURE 1 Schematic representation of the general structure of camel conventional IgG1 and the nonconventional IgG2 and IgG3. The lack of light chain is itself a consequence of the absence of the CH1 domain in the gamma 2 and gamma 3 chains, due to a splicing defect of the CH1 exon.
The figure is from IMGT: Camelidae IgG antibodies (http://www.imgt.org/IMGTbiotechnology/Camel_IgG.html#characteristics) with permission of IMGT®, the international ImMunoGeneTics information system ® (http://www.imgt.org).
References
1. Ciccarese S,et al. The Camel Adaptive Immune Receptors Repertoire as a Singular Example of Structural and Functional Genomics. Frontiers in Genetics. 2019.00997